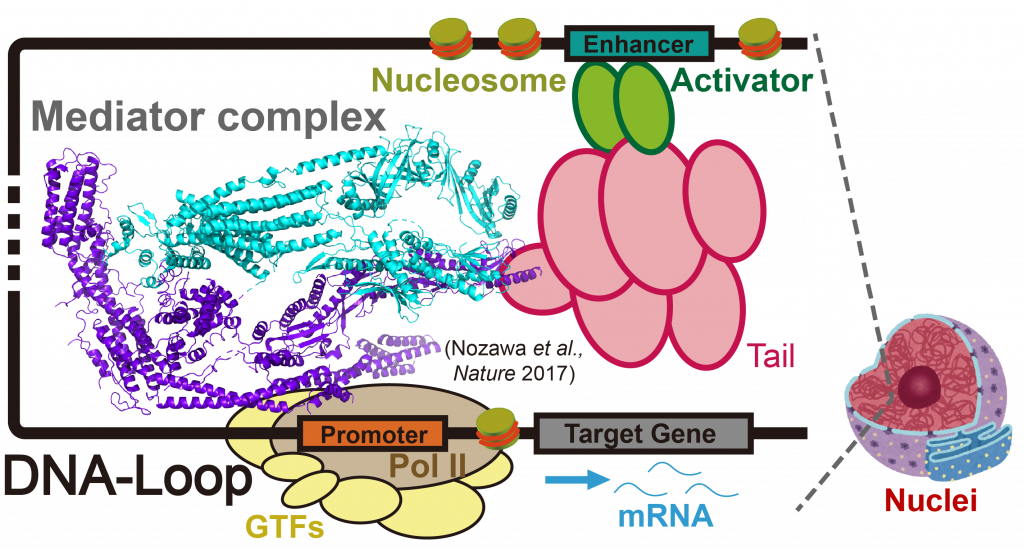
Cell type-dependent gene activation is enabled by the three-dimensional regulation of chromatin conformations, which involves “DNA-loop”. DNA-loop regulates transcriptional activation with the help of a multi-subunit co-activator called Mediator. Mediator directly connects activator which is bound to enhancer DNA with RNA polymerase II on the promoter DNA via a long DNA chain of several Kbps to Mbps, thereby forming the aforementioned DNA-loop structure in chromatin. A number of diseases have been linked to DNA-loop, including cancers, as DNA-loop plays a key role in forming clusters of enhancers, or super-enhancers, that drive unusually high level of expression of genes, such as oncogenes. Hence, DNA-loop may be a potential therapeutic target for selective disruption of oncogene expression. Notably, Mediator seems to have a key role in forming super-enhancer structure. Super-enhancers are clusters of DNA-loop, and they can drive high expression levels of oncogenes in cancer cells. Another interesting regulatory mechanism by Mediator is a formation of liquid-liquid phase separation (LLPS). LLPS represents a phenomenon in which proteins and/or nucleic acids achieve higher local concentrations and form “membrane-less organelles”.
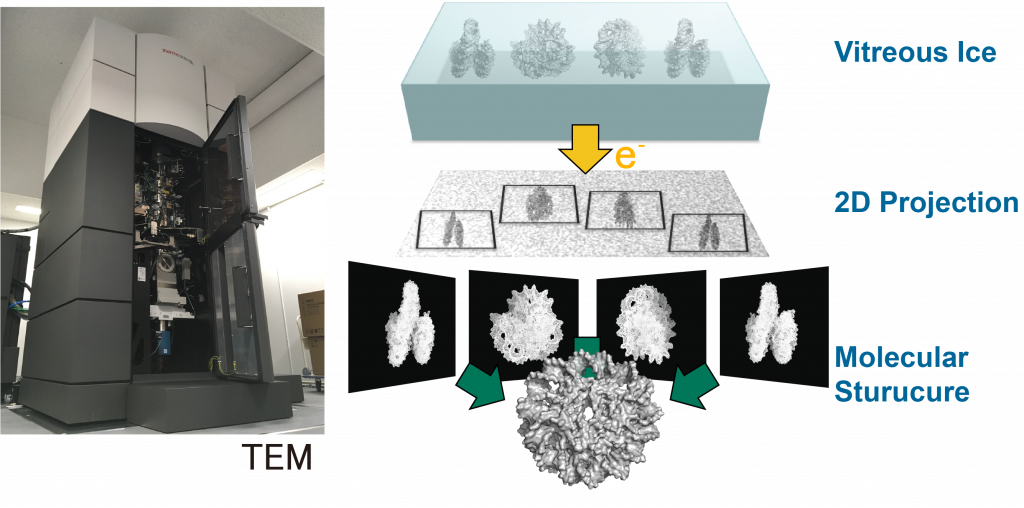
Our laboratory aims to elucidate the atomic structure of DNA-loop using Cryo-electron microscopy and X-ray crystallography, to reveal the molecular mechanism of transcriptional initiation and epigenetic regulation thereof. So far, we established a protein co-expression system for the core Mediator of 15 subunits, which is the smallest functional unit of the Mediator, and performed X-ray crystallographic analysis. The result clarified that it is involved in the transcription initiation of Pol II on the promoter. We are also interested in chromatin structures constituting the DNA-loop. Applying cryo-electron microscopy analysis, we have recently characterized a unique chromatin structural unit H3-H4 octasome that resembles the normal nucleosome but lacks H2A and H2B.